UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of The Securities Exchange Act of 1934
Date of Report (Date of Earliest Event Reported):
(Exact name of registrant as specified in its charter)
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code:
(Former name or former address, if changed since last report.)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered | ||
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
| Item 7.01 | Regulation FD Disclosure. |
On November 15, 2021, Cue Biopharma, Inc. (the “Company”) will be presenting at the Stifel Healthcare Conference. A copy of the Company’s corporate presentation to be made at the conference is attached to this Current Report on Form 8-K as Exhibit 99.1 and is incorporated by reference into this Item 7.01.
The information in this Item 7.01 of this Current Report on Form 8-K, including Exhibit 99.1, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such filing.
| Item 9.01 | Financial Statements and Exhibits. |
d) Exhibits
| Exhibit No. | Description | |
| 99.1 | Cue Biopharma, Inc. Corporate Presentation, dated November 2021 | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Cue Biopharma, Inc. | ||||||
| Date: November 15, 2021 | By: | /s/ Daniel R. Passeri | ||||
| Name: | Daniel R. Passeri | |||||
| Title: | Chief Executive Officer | |||||

Corporate Presentation Immune Responses, On Cue™ Nasdaq: CUE November 2021 Exhibit 99.1

© 2021 CUE BIOPHARMA Forward-Looking Statements Disclosure This presentation has been prepared by Cue Biopharma, Inc. (“we,” “us,” “our,” “Cue” or the “Company”) and is made for informational purposes only and does not constitute an offer to sell or a solicitation of an offer to buy securities, nor shall there be any sale of any securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction. The information set forth herein does not purport to be complete or to contain all of the information you may desire. Statements contained herein are made as of the date of this presentation unless stated otherwise, and neither this presentation, nor any sale of securities, shall under any circumstances create an implication that the information contained herein is correct as of any time after such date or that information will be updated or revised to reflect information that subsequently becomes available or changes occurring after the date hereof. This presentation contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 that are intended to be covered by the “safe harbor” created by those sections. Forward-looking statements, which are based on certain assumptions and describe our future plans, strategies and expectations, can generally be identified by the use of forward-looking terms such as “believe,” “expect,” “may,” “will,” “should,” “would,” “could,” “seek,” “intend,” “plan,” “goal,” “project,” “estimate,” “anticipate,” “strategy,” “future, “vision”, “likely” or other comparable terms. All statements other than statements of historical facts included in this presentation regarding our strategies, prospects, financial condition, operations, costs, plans and objectives are forward-looking statements. Examples of forward-looking statements include, among others, statements we make regarding our development plans for CUE-101, CUE-102 and the continued buildout of our pipeline, the sufficiency of our cash, cash equivalents and marketable securities to support the clinical development of CUE-101 and CUE-102, anticipated results of our drug development efforts, including study results, our expectations regarding the timing of milestone events, regulatory developments and expected future operating results. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, projections, anticipated events and trends, the economy and other future conditions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict and many of which are outside of our control. Our actual results and financial condition may differ materially from those indicated in the forward-looking statements. Therefore, you should not rely on any of these forward-looking statements. Important factors that could cause our actual results and financial condition to differ materially from those indicated in the forward-looking statements include, among others, our limited operating history, limited cash and a history of losses; our ability to achieve profitability; potential setbacks in our research and development efforts including negative or inconclusive results from our preclinical studies, our ability to secure required U.S. Food and Drug Administration (“FDA”) or other governmental approvals for our product candidates and the breadth of any approved indication; adverse effects caused by public health pandemics, including COVID-19, including possible effects on our operations and clinical trials; negative or inconclusive results from our clinical studies or serious and unexpected drug-related side effects or other safety issues experienced by participants in our clinical trials; delays and changes in regulatory requirements, policy and guidelines including potential delays in submitting required regulatory applications to the FDA; our reliance on licensors, collaborators, contract research organizations, suppliers and other business partners; our ability to obtain adequate financing to fund our business operations in the future; our ability to maintain and enforce necessary patent and other intellectual property protection, competitive factors, general economic and market conditions; and the other risks and uncertainties described in the Risk Factors and in Management's Discussion and Analysis of Financial Condition and Results of Operations sections of our most recently filed Annual Report on Form 10-K and any subsequently filed Quarterly Report(s) on Form 10-Q. Any forward-looking statement made by us in this presentation is based only on information currently available to us and speaks only as of the date on which it is made. We undertake no obligation to publicly update any forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise.

Leading the next wave of disruptive, breakthrough immunotherapies addressing the specificity and diversity of the human immune system to cure complex human disease. © 2021 CUE BIOPHARMA Cue Biopharma’s Vision Harnessing natural signals (“Nature’s Cues”) for tailored immune activation against cancers to enhance efficacy and tolerability Exploit evolutionary selectivity of the immune system enabled by rational protein engineering to design therapeutics with potentially enhanced activity Emerging clinical data, including clinical response and patient benefit, provides potential for de-risking and validation of the entire platform Platform modularity and scalability expected to support broad clinical applications

Asia Rights, with option for China sub-license: CUE-101 CUE-102 NEXT ANTICIPATED MILESTONE PARTNER TARGET SELECTION PRE-CLINICAL PHASE 1 CUE-101 (HLA:A02) Monotherapy 2L+ (HPV) CUE-101 + Keytruda 1L (HPV) CUE-102 (HLA:A02-WT1) CUE-302 CUE-103(HLA:A11-KRAS G12V) CUE-101 Neoadjuvant (HPV) Neo-STAT-HLA: A02 Cancer: CUE-100 series and derivatives IL-2 RDI-STAT 1H ‘22: Top-line Phase 1b data 2Q ‘22: Initiate Dose Expansion 2H 2021: Trial Initiated 1Q 2022: IND Filing 2022: IND Enabling Studies 2022: IND Enabling Studies CUE-100 Series: Changing the Therapeutic Landscape Neo-STAT-HLA: A11 & 24 Infectious disease: CUE-200 series CD80 & 4-1BBL CUE-201 Autoimmune disease: CUE-300 series PD-L1 & Undisclosed CUE-301 (Proins / DR4) CUE-302 Autoimmune disease: CUE-400 series IL-2/TGF-β CUE-401 (iTregs) © 2021 CUE BIOPHARMA

Checkpoint inhibitors provided early insights that immunotherapy has the potential to eradicate cancer, however many challenges remain Overall response rates for checkpoints are 15-25%, depending on tumor type Many tumors show an absence of T cell infiltration i.e., many tumors are “cold” How do we make immunotherapy more effective? Increasing and activating tumor targeted T cells is key to enhancing therapeutic benefit IL-2 was the first cytokine to be successfully used in the treatment of cancer because it promotes expansion, function and survival of effector T cells IL-2 was approved for therapy of metastatic melanoma and metastatic renal cell carcinoma Overall response rates have remained low due to narrow therapeutic window and poor tolerability A significant challenge in the development of IL-2 as a therapeutic anti-tumor agent is the indiscriminate activation of numerous immune cells – leading to severe toxicity and narrow therapeutic window © 2021 CUE BIOPHARMA Immunotherapy Has Transformed Oncology Treatment

IL-2 Therapy Challenges: Non-selective Immune Activation = Poor Tolerability and Poor Therapeutic Performance CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ Wild Type IL-2 or IL-2 Variants Lack of Selectivity for Tumor-specific CD8+ T cells Non-specific Activation of ALL CD8+ T cells NON TUMOR-SPECIFIC CD8+ T CELL TUMOR-SPECIFIC CD8+ T CELL CD8+ CD8+ Considerations and Challenges Vast majority of T cells are NOT tumor-specific Need for IL-2 selectivity for tumor-specific T cells Activation of Tregs Toxicities (VLS, CRS etc.) © 2021 CUE BIOPHARMA Examples Wt IL-2 (aldesleukin) “Not-alpha” IL-2 variants Tumor-localized IL-2 variants (e.g., FAP-targeted) “Masked” IL-2 for conditional activation Lineage-biased IL-2 variants (e.g. CD8+ T cells, or PD-1+ T cells) CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+

© 2021 CUE BIOPHARMA CUE-100 Series: Designing an IL-2 Variant in Context of T Cell Receptor (TCR) Engagement TUMOR-SPECIFIC T CELLS +++ IL-2R IL-2 TCR p-HLA IL-2R IL-2 TCR p-HLA +/- NON TUMOR-SPECIFIC T CELLS >>> Fc Fc CUE-100 series Immuno-STAT Engineered IL-2 favors activation of TCR-engaged T cells Abrogate binding to IL-2R⍺ Attenuate binding to IL-2Rβ Selective engagement tumor-specific T cells p-HLA p-HLA (2X) IL-2 variant (4X) IL-2 variant IgG-Fc CUE-100 series Immuno-STAT Fc backbone for stability, manufacturing, and half-life Molecular structure exploits concurrent TCR and IL-2R engagement to activate tumor-specific CD8+ T cells

LEGEND IL-2 IL-2R peptide-HLA TCR IL-2 Immuno-STAT IL-2 p-HLA Fc +++ +++ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ CD8+ Focused Activity on Tumor-specific T cells Biased Activation and Expansion of Tumor-specific T cells (or “TCR-engaged” T cells) ADVANTAGES Selective activity of IL-2 on tumor-specific T cells (cis-interaction) Potential for activation of TCR-engaged anti-tumor T cells (trans-interaction of IL-2) Demonstrated expansion of NK cells (IL-2-mediated) Minimize Treg activation Generally well-tolerated to date lead candidate CUE-101 dosed up to 8.0 mg/kg with no MTD CUE-100 series CUE-100 Series: Directing IL-2 Activity to Tumor-specific T cells and Enhancing Tolerability and Efficacy © 2021 CUE BIOPHARMA NON TUMOR-SPECIFIC CD8+ T CELL TUMOR-SPECIFIC CD8+ T CELL CD8+ CD8+

CUE-100 Series: Opportunity to Maximize the Fullest Potential of IL-2 © 2021 CUE BIOPHARMA Wild Type IL-2 or IL-2 Variants NON TUMOR-SPECIFIC T CELLS TUMOR SPECIFIC T CELLS EQUAL ENGAGEMENT OF ALL T CELLS +++ +++ CUE-100 Series TUMOR-SPECIFIC T CELLS NON TUMOR-SPECIFIC T CELLS BIASED ACTIVATION OF TUMOR-SPECIFIC T CELLS +/- +++ IL-2 IL-2R p-HLA TCR The CUE-100 series has the potential of enabling a broad therapeutic window to enhance IL-2’s clinical effectiveness TOLERATED CLINICAL DOSES 0.037 mg/kg (approved dose) 0.006 mg/kg (RP2D) 0.006 to 0.024 mg/kg Proleukin® (aldesleukin) Bempegaldesleukin (NKTR-214) and nemvaleukin alfa (ALKS-4230) SAR444245 (formerly THOR-707) CUE-101 dosed up to 8.0 mg/kg NO MTD identified LEGEND

Platform Validation CUE-100 Series: Validate, Expand, and Accelerate CUE-100 Series Immuno-STATs CUE-101 (A02): HPV E7 Phase 1 Mono in 2L+ R/M HNSCC Phase 1 CPI Combo in 1L R/M HNSCC Phase 1 Neo-Adj in locally/advanced HNSCC CUE-101 Clinical Data Validates the Immuno-STAT Platform: Clinical and mechanistic PoC for selective IL-2-targeting to tumor-relevant T cells and NK cells Generally well tolerated at efficacious doses PD and PK (lack of evidence of drug-clearing ADAs) Anti-tumor efficacy in monoTx based on RECIST1.1 De-risks CUE-101 as well as the IL-2-based CUE-100 series Currently in RP2D dose expansion Currently in dose escalation Initiated 4Q 2021 Signal 1 HLA-A*02:01 + HPV-16 E711-20 Signal 2 Engineered IL-2 variant © 2021 CUE BIOPHARMA

CUE-101: Lead Clinical-stage Asset from IL-2 based CUE-100 Series © 2021 CUE BIOPHARMA Overview of CUE-101 Phase 1 Monotherapy Part A Dose Escalation Study Overview of CUE-101 Phase 1 Monotherapy Dose Escalation and Expansion Study Phase 1 Part A Dose Escalation Completed and Part B Expansion Enrolling 38 patients with recurrent/metastatic H&N cancer across 7 dose escalation cohorts 15 patients treated at the RP2D (4 mg/kg) PK Dose proportional exposure, sustained exposure with repeat dosing No evidence of anti-drug antibodies (ADAs) PD Expansion of disease-relevant CD8+ T cells and NK cells, with evidence of tumor T cell infiltration Tolerability Patients given CUE-101 doses ranging from 0.06 mg/kg – 8 mg/kg Generally well-tolerated with no MTD identified Efficacy Clinical activity observed across several dose cohorts (partial response/stable disease) PR and durable SD observed at RP2D (4.0mg/kg Q3W)

Data extracted from EDC 03-Nov-2021 (7 patients remain on study) *To qualify as Durable Stable Disease requires SD at ≥2 consecutive post-treatment scans through week 12. Preliminary Tumor Responses for CUE-101 Monotherapy at the RP2D (4 mg/kg) © 2021 CUE BIOPHARMA

CUE-101: Confirmed PR with ~ 54% Reduction in Target Lesions Increase in HPV E7-specific CD8+ T cells with minimal change in total T cells Confirmed PR Duration of Response 30 weeks Patient remains on treatment *Data updated 28OCT21 © 2021 CUE BIOPHARMA Case History Prior therapy: 1L cetuximab 2L pembrolizumab Progressive disease prior to enrollment Treated in metastatic 3L setting with 4.0 mg/kg CUE-101 Q3W (Cohort 6) Patient completed 13 cycles of CUE-101 and remains on study with PR ongoing

CUE-101: Tumor Necrosis and T Cell Infiltrates in Target Lesions © 2021 CUE BIOPHARMA Case History Prior therapy: 1L chemotherapy 2L pembrolizumab Progressive disease prior to enrollment Treated in metastatic 3L setting with 1.0 mg/kg CUE-101 Q3W (Cohort 4) Confirmed and sustained SD through 18 weeks Target lesion resected at 18 weeks due to proximity to an artery Patient remains disease free post resection Immunostaining (CD8+ T cells = rose; PD-LI = brown) Hematoxylin and Eosin Stain

CUE-101: Increase in Tumor Infiltrating T Cells (TILs) 20x Pre-dose Cycle 3 Day 1 CD3 GZMB Cytokeratin CD3 GZMB Cytokeratin IHC staining indicates increase in TILs (CD3+) and granzyme (GZMB) within a target tumor lesion following CUE-101 monotherapy © 2021 CUE BIOPHARMA Case History Prior therapy: 1L chemotherapy 2L pembrolizumab Progressive disease prior to enrollment Treated in metastatic 3L setting with 2.0 mg/kg CUE-101 Q3W (Cohort 5)

Part C: Pembrolizumab Combination Dose Escalation Pembrolizumab 200 mg, Q3W CUE-101 Dose (mg/kg) 4.0 Dose (mg/kg) 2.0 1.0 Abbreviations: PD, pharmacodynamics; PK, pharmacokinetics; RP2D, Recommended Phase 2 Dose Dosing complete, no DLTs Cohort 1 Cohort 3 Cohort 2 Enrolling Dosing complete, no DLTs Part C: Dose Escalation of CUE-101 in Combination with Pembrolizumab Preliminary results: 3 of 3 patients from cohort 2, 2 mg/kg, demonstrated tumor reductions in their target lesions on their first scan after two cycles of therapy All 3 remain on treatment 1st patient has confirmed SD 2nd patient unconfirmed PR 3rd patient has had 1 scan to date Cohort 2 (2mg/kg) © 2021 CUE BIOPHARMA

CUE-101: Potential for Multiple Registration Paths © 2021 CUE BIOPHARMA Monotherapy 2nd line+ therapy for HPV+ head and neck cancer Combination therapy First line HPV+ Head and Neck cancer in combination with pembrolizumab Neoadjuvant therapy Early treatment in neoadjuvant setting (study launched in 2H 21)

Phase 1 Adj in recurrent HNSCC Platform Validation and Expansion CUE-100 Series Immuno-STATs CUE-102 (WT-1) IND filing 1Q 2022 CUE-103 (KRASG12V) IND enabling activities initiated Platform Expansion via CUE-102 and CUE-103: Demonstration of platform modularity Expansion of tumor antigens (WT-1, KRAS) Expansion of HLA allelic coverage (A02, A11) Expansion into major disease indications with significant patient reach Exploits clinical de-risking of IL-2-based CUE-100 series by CUE-101 Safety and tolerability of IL-2 dosing Well defined regulatory strategy Potential for expedited clinical development path CUE-100 Series: Validate, Expand, and Accelerate CUE-101 (HPV E7) Phase 1 Mono in 2L+ R/M HNSCC Phase 1 CPI Combo in 1L R/M HNSCC Phase 1 Neo-Adj in locally/advanced HNSCC © 2021 CUE BIOPHARMA

Top-ranked onco-fetal tumor antigen by the NCI with restricted tissue-expression Broad therapeutic opportunity in numerous solid cancers and hematological cancers Core IL-2 framework is de-risked by the clinical experience of CUE-101 IL-2 Variant (same as CUE-101) Fc Backbone (same as CUE-101) HLA A:02 (same as CUE-101) WT137-45 CUE-102: Targeting Wilms Tumor 1 (WT1) – IND Filing 1Q 2022 Molecular Design © 2021 CUE BIOPHARMA CUE-102 Selectively Expands WT-1-specific T Cells CUE-102 Expands WT-1-specific T Cells that are Cytolytic WT137-45-specific CD8+ T cells (Fold-change over background) WT137-45-specific CD8+ T cells (Fold-change over background) % Specific Lysis

© 2021 CUE BIOPHARMA Broad therapeutic opportunity for targeting of multiple cancers with KRAS driver mutations (NSCLC, CRC, PC, etc.) KRAS G12V is a key driver mutation in highly prevalent solid tumors KRASG12V is a validated epitope that is recognized by anti-tumor T cells KRASG12V A11 asset serves as a beachhead for targeting multiple KRAS mutations (G12D, G12R) and global alleles (i.e., A03, B07) - Source (UPenn PMID 34272369) CUE-103: KRAS G12V – Proof of Concept for Targeting KRAS Mutations Molecular Design IL-2 Variant (same as CUE-101) Fc Backbone (same as CUE-101) KRAS7-16 G12V HLA A:11 Molecular Design CUE-103 Selectively Expands KRASG12V-specific T Cells

Expansion of Modular CUE-100 Series Into Broad Range of Cancers © 2021 CUE BIOPHARMA Sources (Accessed 2020) Annual Incidence: SEER (US), Globocan (EU and China) Antigen Expression: NIH TGCA, Cancer Atlas WT1 HPV KRAS Broad Universe of Addressable TCR Targets with CUE-100 Series Viral antigens (HPV, EBV) Cancer-Testes Antigens (WT1, MAGE) Lineage Antigens (Gp100) Neoantigens (KRAS) Head and Neck Cervical Other Solid (i.e., Ovarian, CRC, Pancreatic, Breast, NSCLC) Hem (i.e., AML, ALL, MM) Lung CRC Pancreatic
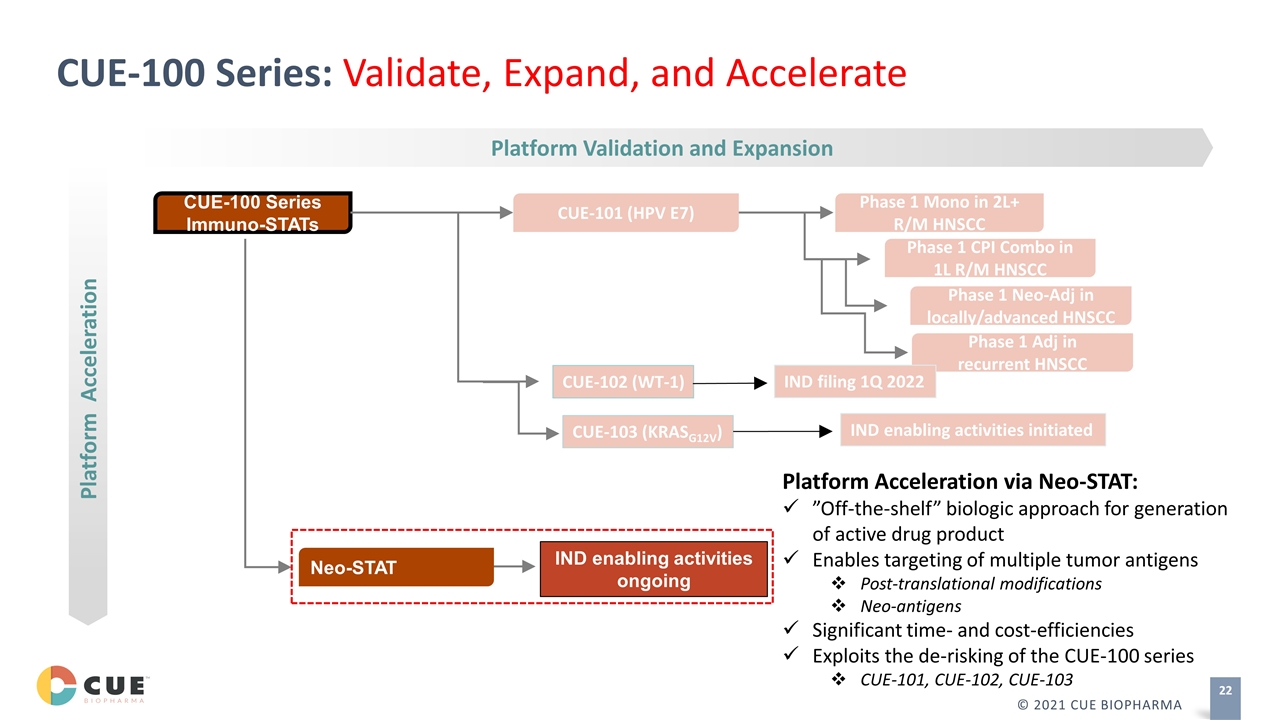
Phase 1 Adj in recurrent HNSCC Platform Validation and Expansion CUE-100 Series Immuno-STATs Neo-STAT Platform Acceleration IND enabling activities ongoing Platform Acceleration via Neo-STAT: ”Off-the-shelf” biologic approach for generation of active drug product Enables targeting of multiple tumor antigens Post-translational modifications Neo-antigens Significant time- and cost-efficiencies Exploits the de-risking of the CUE-100 series CUE-101, CUE-102, CUE-103 CUE-100 Series: Validate, Expand, and Accelerate CUE-101 (HPV E7) Phase 1 Mono in 2L+ R/M HNSCC Phase 1 CPI Combo in 1L R/M HNSCC Phase 1 Neo-Adj in locally/advanced HNSCC CUE-102 (WT-1) IND filing 1Q 2022 CUE-103 (KRASG12V) IND enabling activities initiated © 2021 CUE BIOPHARMA

CUE-100 Neo-STAT (NST): “Off-the-Shelf” Universal Scaffold to Address Tumor Heterogeneity and Platform Scalability © 2021 CUE BIOPHARMA Potential Therapeutic Applications Target multiple tumor antigens on multiple HLA alleles Peptide mixes / Multi-antigen based cocktail therapy Integration of post-translationally modified peptides Extension to cancer neoantigens ➙ Personalized medicine Neo-STAT will allow for significant time- and cost-efficiencies for rapid generation of therapeutic molecules + Empty HLA Stabilized through engineering CUE-100 IL-2 Peptide Conjugation Sites Neo-STAT Rapid conjugation of tumor epitopes CMV-IST CMV-NST MART1-IST MART1-NST Neo-STATs (NST) and Immuno-STATs (IST) Exhibit Comparable Activity NSTs with SARS-CoV2 Epitope Expand CD8+ T Cells from Vaccinated Human Subjects Neo-STAT Manufacturability HLA-A02 HLA-A11 HLA-A24 Production ✓✓ ✓✓ ✓✓ Biophysical Properties ✓✓ ✓✓ ✓✓ Biological Activity ✓✓ ✓✓ Ongoing Initiation of CMC to support IND ✓✓ Tbd Tbd

CUE-101 Clinical Development Monotherapy holds promise of registration path for 2L+ HNSCC patients Clinical data de-risks CUE-101 as well as CUE-100 series Demonstrates attractive PK/exposure and tolerability profile with evidence of clinical anti-tumor activity Combination trial ongoing with pembrolizumab in 1L patients (exploits the potential for synergistic MoA) Neoadjuvant study launched 2H (generate further evidence of TIL expansion and induction of tumor killing) CUE-102 targets Wilms Tumor 1 (WT1) driven cancers – IND filing Q1 2022 Broad opportunity across multiple solid and hematological cancers Preclinical data shows strong human T cell expansion and effector function CUE-103 targeting KRAS G12V mutation – IND enabling activities ongoing KRAS G12V is a key driver mutation in highly prevalent solid tumors CUE-103 serves as a beachhead for targeting other KRAS mutations and additional HLA alleles © 2021 CUE BIOPHARMA Summary of Strategic Developments for CUE 100 Series

Thank you Rationally Engineered Biologics to Restore Immune Balance by Harnessing Nature’s Cues for Selective and Specific Immune Modulation
